Abstract
Introduction: BGB-3111 is a potent, specific and irreversible inhibitor of Bruton's tyrosine kinase (BTK). In its first-in-human study in Australia, New Zealand, South Korea, US and Europe, it was demonstrated that BGB-3111 can achieve complete, continuous BTK occupancy in peripheral blood mononuclear cells and lymph nodes, associated with durable clinical responses in patients (pts) with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) and Waldenström's macroglobulinemia (WM). Here we report the initial findings of a Phase 1 trial of BGB-3111 in Chinese pts with B-cell malignancies.
Patients/ Methods: Chinese pts with relapsed/ refractory B-cell malignancies were enrolled to 1 of 2 dose cohorts of BGB-3111 (320 mg PO QD or 160 mg PO BID). Adverse events (AEs) are reported per CTCAE v4.03, responses per standard criteria according to histology (NHL IWG criteria 2014, CLL IWG criteria 2013, WM IWWM criteria 2013), BTK occupancy determined using an irreversible binding assay in PBMCs.
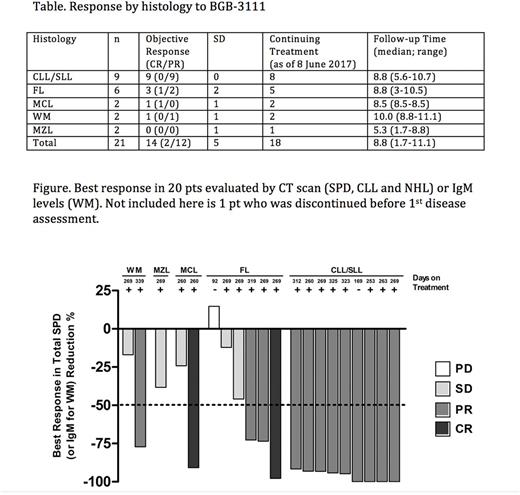
Results: As of 8 June 2017, 21 pts have been enrolled. Median number of prior therapies was 3 (range 1-9). 11 pts received 160 mg PO BID and 10 pts received 320 mg PO QD. The median follow-up time is 8.8 months (range 1.7-11.1 months). All pts were included for safety evaluation and efficacy analyses. Complete BTK occupancy at dosing trough in PBMCs was demonstrated at both dose levels. As of the cut-off date, there was no dose limiting toxicity (DLT). The most frequent grade 3 or above AEs (≥ 10%) were neutropenia (28.6%), thrombocytopenia (14%), leukocytosis (14%), and lymphocytosis (14%). 4 SAEs have been reported, including one event each of toxic epidermal necrolysis (G4), febrile neutropenia (G3), neutropenia (G4), and thrombocytopenia (G4). One MZL pt was discontinued due to G4 toxic epidermal necrolysis and two pts (1 CLL and 1 FL) were discontinued due to disease progression after 169 days (CLL) and 92 days (FL) of treatment. One death (one FL pt) was reported after drug discontinuation due to disease progression. There were no major bleeding and atrial fibrillation reported. Response to treatment by histology is summarized in Table 1 and Figure 1. As of the cut-off date, 18/21 pts remain on study treatment.
Conclusions: BGB-3111 is well tolerated and highly active in Chinese pts with B-cell malignancies. The BTK inhibition and anti-tumor activity and safety profile of BGB-311 in Chinese patients are similar to those profiles we have seen in the previously reported data for BGB-3111. Several Phase 2 studies in different indications, including CLL/SLL, MCL, WM, and non-GCB DLBCL, have been initiated in China to further evaluate the efficacy and safety of BGB-3111 in Chinese pts.
Xue: BeiGene: Employment. Yang: BeiGene: Employment. Zhang: BeiGene: Employment. Wang: BeiGene: Employment. Huang: BeiGene: Employment. Feng: BeiGene: Employment.
Author notes
Asterisk with author names denotes non-ASH members.